Stephen A. Levine, Ph.D.
I became interested in Phosphatidylserine after hearing Parris Kidd, Ph.D. lecture in 1996. As I reviewed the literature, the applications were impressive, with broad anti-aging effects, memory boosting, and mood enhancement in normal individuals. There are specific benefits for patients with Minimal Memory Disorder (MMDI), Alzheimer's, Parkinson's disease, and depression. The dosage used in almost all double-blind studies is 300 mg per day of Phosphatidylserine taken over a two or three month period. In some cases, even higher dosages are utilized with apparent further benefit and over longer periods of time. To my knowledge, no serious side effects have been reported. I have reviewed almost 20 double-blind clinical trials, and other human studies. Most of the important studies were done in the late 1980's treating Alzheimer’s disease, cognitive decline in aging, dementia, mental impairment and mood in aged patients, in depressive disorders, in inhibition of the stress response, and in patients with arteriosclerotic disease. I will review salient points to highlight the potential broad ranging benefits from Phosphatidylserine administration.
Until recently, the vast majority of studies utilized Bovine Cortex-derived Phosphatidylserine (BC-PS) (Fidia pharmaceutical grade). Presently, the use of bovine brain material has come into disfavor. The use of plant Phosphatidylserine from soy (SPS) does not pose a risk to patients in developing prion infection. So far, four studies that we know of have been performed with S-PS. The study supports previous research with BC-PS, also confirming that PS is the active agent in the previous studies and not some unknown "carry along" compound. This study, by Gindin et al., stands out because two additional parameters of clinical interest were noted. The patients were asked if they would continue to use PS even if they had to pay for it, and their positive response strongly supported the efficacy of the compound, as will be discussed. Secondly it was observed that PS prevented depressive symptoms associated with winter depression (see figure 1) This was a statistically significant finding and it expands the spectrum of benefits now associated with PS to include Seasonal Affective Disorder.
<table border="0" cellpadding="6" cellspacing="6" width="100%"> <tbody><tr> <td align="center">Depression Symptoms Score
Placebo (N=26) P< (N=31) P<0.4</td> </tr> </tbody> </table> <table cellpadding="6" cellspacing="0" width="100%"> <tbody><tr> <td width="50">Summer</td> <td>
</td> <td width="50">Winter</td> </tr> <tr> <td bgcolor="#000000" width="50">
 </td> <td>
</td> <td> </td> <td bgcolor="#0099ff" width="50">
</td> <td bgcolor="#0099ff" width="50"> </td> </tr> </tbody> </table>
</td> </tr> </tbody> </table> 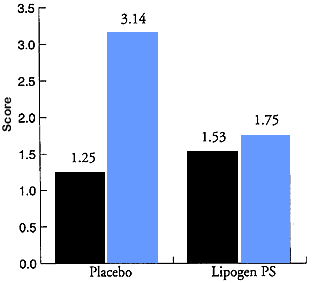
In a 1988 article, (Amaducci L, and the SMID Group), it was found that among the severely impaired patients, or those on the placebo over a six-month period, all parameters declined, while those treated for the first three months with PS improved during the second period. Some of the effects were mild in early treatment, however, six months after the beginning of treatment, the effects were detected on a wider range of measures and were more clearly apparent. These findings suggest that PS may prevent further cognitive decline in Alzheimer's dementia (AD) patients, and the effects may persist beyond the period of administration.
In a study done in 1986, (Delwaide PJ, et al.), a double-blind format was used to study the effects of PS in senile demented (SD) patients. The results at the end of the study were the same as those obtained three weeks later. The types of behaviors that were most modified included dressing, feeding, bowel and bladder control, ability to go to the toilet unaided, interpersonal relations, relationship to the environment, behavioral problems, and verbal expression. In the treatment group, a positive difference was seen for all 10 categories whereas in the placebo group, deterioration was seen in four categories. This data may indicate the kind of overall benefit in quality of life, which may be achieved.
A double-blind placebo-controlled trial was conducted with BC-PS in elderly patients with chronic cerebrovascular disease (Villardita C., et al., 1987). At the standard dosage of 300 mg, the compound was not significantly better than the control in psychometric parameters, as demonstrated by EEG and in clinical status. However flicker fusion frequency improved both after rest and after mental activity. The lack of cognitive effects stands out in this study. However, only a few cognitive functions were assessed and were normal initially, whereas the flicker function test was below the normal range, and was normalized by the treatment.
Senile mental deterioration was studied in three clinics in Italy, using BC-PS over a 60-day period in double-blind placebo format (Palmieri, G., et al., 1987). They concluded that BC-PS appears to exert relevance of the effects in two contexts; one relating to cognitive functions of vigilance, attention, and short-term memory, and the other relating to behavioral aspects such as apathy, withdrawal and daily living. According to the article, "the observed effects may not only improve the quality of life of these patients, but may also contribute to keeping them within their own families and social background".
In 1989, (Liss, Alan R., 1987), a double-blind study was reported. The test group was less depressed and less anxious than the placebo group. In this case a subgroup of patients with more severe motor disturbances responded more significantly. IV injection of BC-PS produced obvious clinical response within six hours after injection.
A double-blind study in 1992 evaluating early Alzheimer's dementia was published in Europe (Engel, RR. et al.). Using BC-PS on 33 patients, a small but significant improvement, according to clinicians global judgment occurred. EEG mapping indicated global normalization and simple reaction time improvement. The GBS dementia rating scale (and pyschometric test scores) did not change significantly The clinicians’ global rating score indicates an improvement in patients overall functioning.
In a 1993 study, 494 patients from 23 geriatric or general medicine units in Italy were studied (Cenacchi, B., et al.) using BC-PS. The results indicated that the BC-PS group compared to the placebo group showed significantly improved behavior, such as increased motivation, initiative, interest in the environment, and socialization, which were present to various extents in patients upon admission. The study noted that “the resulting improvements in adaptability to the environment can have an important impact on the quality of life of such patients.” The administration of BC-PS together with other drugs failed to show any pharmacological interactions, as judged by the lack of clinical signs and symptoms. This was a large multi-center study that provides impressive stature to the research.
In a 1991 study, (Crook, TH. et al.), using the standard three month, 300 mg dosage of BC-PS, significant differences were noted in 6 out of 20 parameters. All significant differences favored BC-PS. Each of the differences favoring the BC-PS were during the final treatment period (Week 12), and were lost beyond one month after termination of treatment (Week 16). These observations strengthen the experimental conclusion of benefit by BC-PS. Benefits were found in a number of outcomes, such as important tasks of daily life, plus learning and recalling names, faces, and numbers. "Persons most likely to benefit were those above the range of cognitive performance associated with dementia disorders such as Alzheimer's disease, but performed in the low range of normality," is quoted from the study.
"Hence, the compound may be valuable as a prophylactic treatment for Alzheimer's and related dementia-inducing disorders". Some additional benefits on psychometric testing (neuromotor skills) were not significant until after the end of the 12-week period, although clinical benefits were observed after the first three weeks of treatment.
The cognitive effect and behavioral symptoms of BC-PS were studied in 10 elderly women with depressive disorders in which BC PS was administered. Increased brain turnover of noradrenaline, dopamine, acetylcholine and cAME, as well as induced accumulation of glucose were noted. The depressive symptoms in the patients were marked before treatment and after placebo, and were significantly reduced by BC-PS therapy. The HRSD (Hamilton Rating Scale for Depression), as it relates to anxiety, revealed elevated basal scores that did not change after placebo and improved significantly after treatment. The remission in symptoms was clear-cut, based both on clinical observations by medical and paramedical staff, as well as by the analysis of HRSD scores. In particular, drive interests and socialization were increased as described, "the improvement in long-term memory was particularly significant in our patients", and it appeared that learning was also improved.
Phosphatidylserine also appears to protect against the stress response and to reduce anxiety, as demonstrated in previous studies. In a 1990 study (Monteleone, P., et al.), the response of BC-PS to physical stress was tested in eight healthy men who underwent three experiments with a bicycle ergometer in a double-blind format. The physical stress from exercise induced a clear cut increase in plasma epinephrine, norepinephrine, ACTH, cortisol, growth hormone, and prolactin, whereas pretreatment with 50-75 mg of BC-PS significantly blunted the (ACTH and cortisol response to the exercise. Plasma growth hormone and prolactin responses to physical stress were not affected by BC-PS.
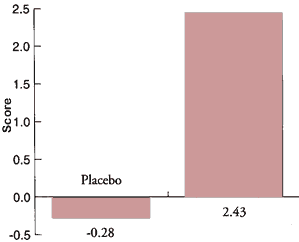
In 1995, a 2-month treatment study (Gindin, J. et al.) using plant-derived phosphatidylserine (S-PS) showed positive effects on daily functioning, emotional state and self-reported general condition of Alzheimer's disease patients. (See figure 2) In a post-trial consumption survey, nearly half of the participants of the treatment group decided to continue treatment at their own expense, in contrast to none in the placebo group. These results of a short-term treatment which had no negative side effects encourages the use of S-PS with AD patients in order to evaluate on an individual basis possible improvement in patient condition. These results also encourage further investigation into long-term treatment and on larger groups with regard to the patient's AD status, i.e. early, mild and severe AD.
<table cellpadding="3" cellspacing="3" width="100%"> <tbody><tr> <td align="center">Memory Test Score
Wechsler >68, P<0.03</td> </tr> </tbody> </table>
- The capsules disintegrated after 30 minutes.
- The PS was well tolerated by the body.
- The basal serum level was reached after 180 minutes from intake.
- No side effects were reported.
PS Levels in Blood Serum
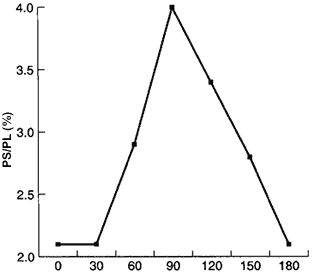
Time (min)
References
Amaducci L, and the SMlD Group. Phosphatidylserine in the treatment of Alzheimer's disease: results of a multicenter study. Psychopharmocol Bull. 24:130-134, 1988. Cenacchi, B., et al., Cognitive decline in thc elderly: a double-blind placebo-controlled multi-center study on efficacy of phosphatidylserine administration. Clin Exp. Rcs. 5:123-133, 1993.
Crook, TH. et al., Effects of phosphatidylserine in age-associated memory impairment Neurol. 41:644-649, 1991.
Crook, TH, et al., Effects of phosphatidylserine in Alzheimer’s disease, Psychopharmacol. Bull., 28:61-66, 1992.
Delwaide PJ, et al. Double-blind randomized controlled study of phosphatidylserine in demented patients. Acta Ncurol Scand, 73: 136-140, 1986.
Engel, RR. et al. Double-blind cross-over study of phosphatidylserine vs. placebo in subjects with early cognitive deterioration of the Alzheimer type. Eur Ncuropsychopharmacol. 2: 149-155, 1992.
Funfgeld, EW, et al., Double-blind study with phosphatidylserine (PS) in Parkinsonian patients with senile dementia of Alzheimer's type (SDAT), Progr. Clin. Biol. Rcs. 317:1235-1246, 1989.
Gindin, J., et al. 1990, Effect of Soy Lecithin Phosphatidylserine (PS) Treatment on Daily Functioning and Self-Reportcd General Condition in Patients with Alzheimer's Disease, The Geriatric Institute of Education and Research Kaplan Medical Centre, Rehovot, and Hadassah Medical School, Hebrew University of Jerusalem, Israel.
Interdiscipl. topics Gerontol 15: 34-53, 1979; ACTA Psychiatr Scand 81: 265-270, 1990; Neurobiol Aging 5: 323-333,1984.
LifeSci. 23: 1093-1102, Clin Trials J. 24 9-17 1987; Neurobiol Aging 8: 403407, 1987.
Liss, Alan R, Copyright, Alzheimer’s disease and Related Disorders 1235-1246, 1987.
Maggioni M., et al. Effects of phosphatidylserine therapy in geriatric patients with depressive disorders. Acta Pyschiatr. Scand 81:265-270, 1990.
Manfredi, M., et al., Risultati clinici della fosfatidil-serina in 40 donne affete da turbe psico-organiche, in eta climaterica e senile. La Clinica Terapeutica, 120:33-36 [English summary], 1987.
Masturzo, P., et al., TSH circadian secretions in aged men and effect of phosphatidylserine dosing. Chronobiologia 17:267-274, 1990.
Monteleone, P., et al., Effects of phosphatidylserine on the neuroendocrine response to physical stress in humans. Neuroendocrinol, 52:243-249, 1990.
Nerozi, D., et al., Fosfatidilsetina e disturbi della memoria nell-anziano, La Clinica Terapeutica, 120:399404 [English summary], 1989.
Nerozzi, D., et al., Early cortisol escape phenomenon reversed by phosphatidylserine (Bros*) in elderly normal subjects. Clin. Trials J. 26:33-38, 1989.
Nizzo, MC., et al., Brain cortex phospholipids liposomes-effects on CSF HVA, 5-HIAA and on prolactin and somatotropin secretion in man. J. Neural Transmission, 43:93-102, 1987.
Palmieri, G., et al., Double-blind controlled trial of phosphatidylserine in patients with senile mental deterioration. Clin. Trials J. 1987, 24:73-83, 1987.
Puca, FM, et al., Exploratory trial of phosphatidylserine efficacy in mildly demented subjects, Clin. Trials J., 24:94-98, 1987.
Ransmayr, G., et al., Double-blind placebo-controlled trial of phosphatidylserine in elderly patients with arteriosclerotic encephalopathy, Clin. Trials J. 24:62-72, 1987.
Shinitsky, M, Ph.D., Kinetics and Safety of Soy Lecithin Phosphatidylserine (PS) Absorption, Weizmann, Institute of Science Rohovot, Israel, 1996 (Study Report, September 1, 1999).
Sinforiani, E., et al., Cognitive decline in aging brain: therapeutic approach with phosphatidylserine, Clin. Trials J. 24:115-124, 1987.
Villardita C., et. al., 1987. Multicenter clinical trial of brain phosphatidylserine in elderly patients with intellectual deterioration. Clin. Trials J. 24:84-93.
The statements made herein have not been evaluated by the U.S. Food and Drug Administration. The products are not intended to diagnose, treat, cure, or prevent any disease.



